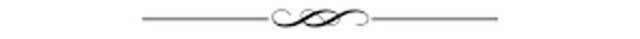Nearly all medical product recalls are voluntarily issued by firms, instead of mandated by the FDA. wavebreakmedia / shutterstock.com
Nearly all medical product recalls are voluntarily issued by firms, instead of mandated by the FDA. wavebreakmedia / shutterstock.com
From the valsartan blood pressure drug contamination that exposed thousands of patients to cancer-causing impurities, to a massive pacemaker recall undertaken to fix a hazardous software bug in half-a-million cardiac devices, health care product quality problems are ever-present and highly dangerous.
In fact, medical product recalls – in particular severe, life-threatening pharmaceutical drug and medical device recalls – have increased steadily over the last decade.
It is no surprise that medical product recalls are universally negative events. Firms seek to avoid them, customers despise them, and federal regulators are forced to oversee them. They are associated with millions of dollars of unwanted corporate costs and stock price declines, along with substantial and costly regulatory oversight each year.
I spent over a decade as a manufacturing manager in Fortune 500 medical device firms, making scores of recall decisions and recommendations – some good decisions, some less so – and the last several years as an academic researcher committed solely to recall research. My colleagues and I have closely studied what causes defects that lead to recalls, as well as biases that exist in managers’ decisions to recall.
Excessive cost competition
In capitalistic markets, competition is viewed as a force for good. Competition can lower costs, increase access, and hopefully improve quality. These benefits attributed to competition explain the steady call for greater access to generic drugs.
While it is seldom discussed in the crowded debate on health care costs, there may be a downside to the never-ending call for cheaper generic drugs and more affordable care.
Research conducted with my colleagues Rachna Shah from the University of Minnesota and Kaitlin Wowak from the University of Notre Dame shows that intense generic drug competition leads to an increase in severely hazardous manufacturing-related drug recalls.
Our study, published in May 2018, shows that generic drug competition causes firms to cut corners in their manufacturing quality control practices in an effort to remain profitable, leading to an increase in life-threatening drug defects requiring a recall.
One of many examples of this type of problem is the recent Ranbaxy atorvastatin recall. Lapses in the manufacturing quality control system led to contamination from unapproved raw materials. The generic drug manufacturer agreed to a US$500 million government fine.
 Medical product recalls are up over the last decade. Atsushi Hirao/shutterstock.com
Medical product recalls are up over the last decade. Atsushi Hirao/shutterstock.com
Familiar FDA inspectors
One way to help mitigate such unfortunate side effects of competition is through U.S. Food and Drug Administration regulations and quality control practices.
A key tool used by the FDA to improve product quality are plant inspections. FDA plant inspectors visit plants on a two-year rotating cycle. These inspection outcome ratings can be an early warning of future recalls from the plant – if the FDA inspector accurately captures the relevant risks.
In a 2017 study with Shah and Enno Siemsen from the University of Wisconsin-Madison, we found that FDA plant outcome ratings can be used to predict future recalls from products made at that plant. However, this is only true when the inspector has never visited the plant before.
Undue familiarity between the FDA inspectors and plant management weakens the accuracy of the ratings, even after just one repeat inspection. Inspectors become complacent as they become more familiar with the plant and the people who work there.
We found that rotating in a new inspector for each FDA plant inspection would significantly improve the value of these inspections and cost the FDA less than $1 million annually. A small price to pay for medical device safety.
Managerial biases
While regulatory oversight may help reduce defective products requiring a recall, another important dimension to the recall phenomenon are the managers who decide to recall a product.
Intriguingly, nearly all medical product recalls are voluntarily issued by firms, instead of mandated by the FDA. The voluntary nature of these recalls gives managers a high level of discretion in the recall decision.
I worked with Shah and Karen Donohue at the University of Minnesota to study managerial biases in recall decisions made by real industry managers.
One bias relates to the physicians who purchase medical devices on behalf of patients. If managers know that their physician customers are likely to detect the defect in the device before using the product on the patient, then managers are surprisingly less likely to recall. They unconsciously trust the physician to screen out detectable defects, obviating the need for a recall.
This bias was unknown to the managers who participated in this study. The firms that we worked with used these results to train decision-makers to be aware of this unwanted bias.
In the same study, we conducted a behavioral cognition test on managers before they made their recall decision. This three-question test measures whether a person makes decisions based upon either intuition or reflection.
This test was highly explanatory of how a manager made the recall decision. Reflective managers recall much less frequently, as they may tend towards “analysis-paralysis,” seeking excessive amounts of data before choosing to recall. This may explain why, in settings where reflective managers are making recall decisions, firms appear to delay recalls, even when doing so puts customers at an increased risk of harm.
 Physicians sometimes catch a defect in the device early on, before it’s ever used on a patient. Peter Porrini/shutterstock.com
Physicians sometimes catch a defect in the device early on, before it’s ever used on a patient. Peter Porrini/shutterstock.com
Other causes
Several different co-authors and I have other exciting recall studies underway that seek to continue to dissect this important problem, particularly from the perspective of managerial biases.
For example, one working paper finds that medical product firms with boards of directors that have at least one woman on the board make recall decisions much more effectively and faster than firms with all-male boards.
Another working paper finds that new CEOs at consumer products firms appear to scapegoat the previous CEO. New CEOs tend to announce several recalls early in their tenure, when the previous CEO is likely to be blamed for the product quality problems.
Because product recalls are pervasive and often associated with consumer harm, I sincerely hope that rigorous research continues to unravel this complex enigma in an effort to help firms, regulators and consumers.![]()
About The Author
George Ball, Assistant Professor of Operations and Decision Technologies, Kelley School of Business, Indiana University
This article is republished from The Conversation under a Creative Commons license. Read the original article.
Related Books
at InnerSelf Market and Amazon