Super-tiny nanomaterials are poised to revolutionize our technologies. As for what else they might do to us: nobody really knows.
Every age has its wonder materials. For the Victorians, it was rubber. In the 20th century, it was plastic. And for the digitized 21st century, it may well turn out to be graphene.
Have you heard of graphene? If not, you soon will. It’s one of the newest nano-scaled materials to have emerged from our laboratories—and as the New York Times recently announced, “it’s expected to transform almost every aspect of life.”
Every day, scientists are learning new things about this amazing nanomaterial. But not everything they’re learning about it is a reason for unbridled optimism. Like other wonder materials of the past, graphene may turn out to be not quite-so-wonderful.
What is Graphene?
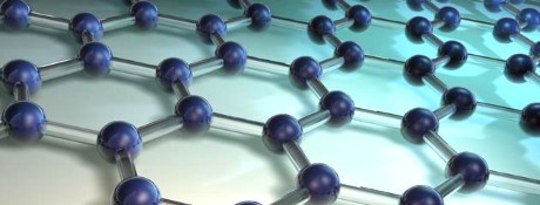
Graphene is derived from graphite, the same carbon-based substance that we put inside pencils. But there’s nothing common about graphene. Its atoms are linked in the thinnest imaginable layer: a honeycomb lattice that’s just one atom thick.
A sheet of graphene is so thin, in fact, that according to the American Chemical Society, one ounce of it could cover 28 football fields; a small chunk is so lightweight that you can balance it on a daisy without bending the petals. It’s also (not incidentally) stronger than steel, harder than diamonds, and more conductive than copper or silicon—in addition to being waterproof, transparent and incredibly pliable.
You’d be hard pressed, in other words, to find another material packed with so many useful properties. No wonder researchers across a wide range of industries are scrambling to bring graphene out of our labs and into our lives. Sales, which were at just under $9 million in 2012, are expected to jump 14-fold to $126 million by 2020, according to Lux Research, a firm that analyzes emerging technologies.
Reasons To Be Wary Given The Lessons Of The Past
Tech experts are predicting that graphene could transform a vast array of consumer products, from condoms to computers to chemical sensors. “5 Reasons Graphene Will Change Your Gadgets Forever,” NBC News blared, with a list that suggested a future filled with paper-thin smartphones, flexible computer displays, medical devices that can “talk” to human cells, and super-long-lasting batteries.
Graphene’s ardent champions claim that it could be used to make stronger and more lightweight cars, vastly more efficient solar cells, even synthetic blood.
The unabashed hype has a familiar ring to it—and it beckons us to be wary, given the lessons of the past. Asbestos was once touted as “the magic mineral” for its ability to withstand flames; only later did we discover that it is, in fact, a killer dust.
Same story with DDT, which proved intensely harmful not only to the disease-carrying mosquitoes it was intended to kill, but also to wildlife (especially birds) and humans; with vinyl, which was ultimately shown to be a significant hormone disruptor; or with the non-biodegradable polyethylene film that has ignited countless ban-the-bag drives. Each was avidly embraced and became widely used before we discovered it had a dark, dangerous side.
 The Collingridge Dilemma
The Collingridge Dilemma
There’s actually a term for this phenomenon, I recently learned: the Collingridge Dilemma. Named for David Collingridge, the otherwise obscure British professor who first postulated it in a 1980 book, it acknowledges the difficulty of predicting the negative impacts of a new technology until such time as that technology has become widely used—by which point, of course, it becomes that much harder to address those negatives, and to impose appropriate remedies. Or, as Collingridge himself put it in The Social Control of Technology,
“When change is easy, the need for it cannot be foreseen; when the need for change is apparent, change has become expensive, difficult and time consuming.”
Which may explain why, when it comes to nanomaterials, researchers are trying to get ahead of the curve. A number of research programs have sprung up to study the issues posed by nanotechnology. For instance, since 2008, both the Environmental Protection Agency and the National Science Foundation have been pouring tens of millions of dollars into a joint program at the University of California Los Angeles and Duke University known as the Center for the Environmental Implications of Nanotechnology (CEIN).
Researchers are pursuing the kinds of questions that, in the past, often didn’t get asked until it would have been too late to address any serious issues that arose from the answers. Can these new materials get into our environment? What happens if they do? How do they interact with different plants and organisms? Do they have toxic effects?
Among other things, these researchers have been testing different nanomaterials in human and animal cells, observing their effects in lab animals, studying how they behave in soil and water, and analyzing the adequacy of existing laws regarding their control and regulation. Thanks to such efforts, “we’ve been generating far more data than I ever thought we would seven years ago,” says Andrew Maynard, director of the University of Michigan Risk Science Center, who has long warned about the need for better oversight of nanotechnology. There are still many important unanswered questions, he says, but “we’re starting to get a real handle on what is really worrisome and what is not quite so worrying.”
Hazardousness Can Take Many Forms
For starters, it’s becoming increasingly clear that some types of nanomaterials pose little environmental or public-health risks, some pose more, and some—especially the newer ones—are still basically question marks. The most hazardous of them would seem to be certain nanomaterials derived from silver, copper, or zinc—all of which easily dissolve in water as well as cells, releasing toxic metals as they do so. But hazardousness can take many forms.
The particular morphology of a nanomaterial makes a big difference. Some (including graphene) have sharp edges that can slice through cell walls. Needle-like carbon nanotubes can act very much like asbestos when inhaled, greatly damaging lung tissue.
As the latest nano-wonder to hit the scene, graphene has only now just begun to draw researchers’ attention. And the first findings have already turned up some worrisome signs, underscoring just how important these early investigations can be. In one 2013 study, for example, Brown University engineers found that graphene sheets with sharp edges could puncture—and possibly penetrate—human skin, lung, and immune cells.
Can We Launch The Graphene Revolution Without Jeopardizing Our Health Or The Environment?
In another recent study, CEIN-affiliated scientists at the University of California–Riverside found that graphene oxide nanoparticles displayed a troubling environmental persistence in certain aquatic settings. In a simulation of an aquifer, they appeared to sink into the sediment, where they were likely to biodegrade. But in the simulation of surface water, such as a lake or stream, the particles tended to glom on to dead leaves and other organic matter. Floating in the water column, they were that much more likely to be absorbed by aquatic micro-critters—or to get into our water supply.
But there’s a distinction to be drawn, of course, between potential risks and actual hazards. As Sharon Walker, one of the authors of the UC–Riverside study, told me,
“...we’re not wanting to raise red flags so much as to inform people, so that red flags don’t have to be raised.”
Such studies present manufacturers and policymakers with an early—and immensely valuable—opportunity to figure out ways of launching the graphene revolution without jeopardizing our health or the environment.
I imagine David Collingridge would be pleased.
This article originally appeared in OnEarth
About the Author
 Susan Freinkel is the author of Plastic: A Toxic Love Story and American Chestnut: The Life, Death, and Rebirth of a Perfect Tree. She has also written for the New York Times, Discover, Smithsonian, Mindful, and other publications. Her interests run wide: she's covered stories ranging from mad cow disease to a vitamin treatment for bipolar disorder, from adoption to the case for zoos to the quest to develop a blue rose. A story about a disease plaguing California oak trees led to her first book, American Chestnut: The Life, Death and Rebirth of a Perfect Tree. It won a 2008 National Outdoor Book Award. After immersing herself in the natural world for that book, she turned her attention to the unnatural world for her next book, Plastic: A Toxic Love Story.
Susan Freinkel is the author of Plastic: A Toxic Love Story and American Chestnut: The Life, Death, and Rebirth of a Perfect Tree. She has also written for the New York Times, Discover, Smithsonian, Mindful, and other publications. Her interests run wide: she's covered stories ranging from mad cow disease to a vitamin treatment for bipolar disorder, from adoption to the case for zoos to the quest to develop a blue rose. A story about a disease plaguing California oak trees led to her first book, American Chestnut: The Life, Death and Rebirth of a Perfect Tree. It won a 2008 National Outdoor Book Award. After immersing herself in the natural world for that book, she turned her attention to the unnatural world for her next book, Plastic: A Toxic Love Story.
Recommended book:
Plastic: A Toxic Love Story
by Susan Freinkel.
 Plastic built the modern world. Where would we be without bike helmets, baggies, toothbrushes, and pacemakers? But a century into our love affair with plastic, we’re starting to realize it’s not such a healthy relationship. Plastics draw on dwindling fossil fuels, leach harmful chemicals, litter landscapes, and destroy marine life. As journalist Susan Freinkel points out in this engaging and eye-opening book, we’re nearing a crisis point. We’re drowning in the stuff, and we need to start making some hard choices. The author gives us the tools we need with a blend of lively anecdotes and analysis. Plastic points the way toward a new creative partnership with the material we love to hate but can’t seem to live without.
Plastic built the modern world. Where would we be without bike helmets, baggies, toothbrushes, and pacemakers? But a century into our love affair with plastic, we’re starting to realize it’s not such a healthy relationship. Plastics draw on dwindling fossil fuels, leach harmful chemicals, litter landscapes, and destroy marine life. As journalist Susan Freinkel points out in this engaging and eye-opening book, we’re nearing a crisis point. We’re drowning in the stuff, and we need to start making some hard choices. The author gives us the tools we need with a blend of lively anecdotes and analysis. Plastic points the way toward a new creative partnership with the material we love to hate but can’t seem to live without.
Click here for more info and/or to order this book on Amazon.