You see it in commercials every day: creams and lotions to reduce wrinkles, dyes to eliminate gray hair, and remedies to reduce muscle and joint aches. Along with these surface level changes, getting older also affects the internal physiology of the body, including increasing inflammation in the brain (Czirr & Wyss-Coray, 2012), degeneration in the retina (Hoh Kam et al, 2010), and permeability of the intestinal walls (Ma et al, 1992). Many industries are built with the goal of reversing the signs of aging. But is there a way to counteract the effects of aging in the body on a deeper level than dyeing your hair? One group of scientists suggests a unique way to turn the clock back on aging-related consequences in the brain using fecal microbiota transfer (FMT; Parker et al., 2022).
FMT uses the principles of parabiosis (See a realted Knowing Neurons article here!) to swap gut microbiomes, defined as the entirety of bacteria and microorganisms living in the healthy gut (Sommer et al, 2013), between aged and young mice. In order to test their hypothesis that using FMT to alter the gut microbiome changes inflammation in the brain and body, Parker and colleagues used a mouse model with 3-month-old mice (young mice) and 24-month-old mice (aged mice). Before the experiment started, the researchers first collected fecal matter to establish the baseline for young and aged mouse microbiomes. Afterwards, mice were given antibiotics for three days to reduce the bacteria present in their guts. After the antibiotic treatment, researchers collected another fecal sample. Following these intial steps, two rounds of FMT were performed, where liquified feces were given nasally and mice were placed in cages containing feces according to their experimental group. The experimental groups in this study were aged mice receiving FMT from young mice and young mice receiving FMT from aged mice, while the control groups were young mice receiving FMT from other young mice or a non-fecal control solution (called young control mice) and aged mice receiving FMT from other aged mice or a non-fecal control solution (called aged control mice). After FMT, feces was collected five days and two weeks later. This experimental design enabled the investigators to study how the age of the gut microbiome affects processes in the brain, retina, and intestines.
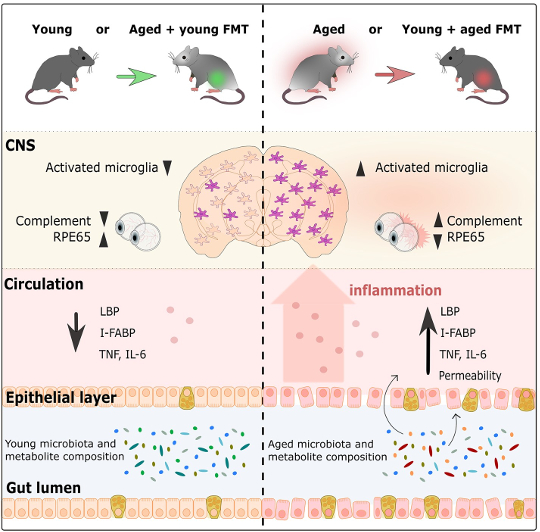
Graphical abstract from Parker et al., 2022
…infusing an aged mouse with a young microbiome undoes the immune response seen with age.
Researchers first investigated how FMT affects the inflammatory response of microglia, the brain’s resident immune cells, in the cortex and corpus callosum (a huge bundle of neurons that allows the two sides of the brain to communicate with one another) (Heneka et al, 2019; Erny et al, 2015). Aged control mice had more activated microglia than young control mice, which reflects the process of normal aging. However, aged mice with young microbiomes had much less microglia activation than aged control mice. Surprisingly, the microglia response was quite similar to what was observed in young control mice. This same pattern was also shown in the opposite direction, as young mice with aged microbiomes had much more microglia activation than young control mice, similar instead to the levels of activation seen in aged control mice. This shows that the age of the microbiome influences the immune response in the brain, and that infusing an aged mouse with a young microbiome undoes the immune response seen with age. Similarly, giving a young mouse an aged microbiome accelerates age’s effect on the brain’s immune cells.
…the microbiome impacts age-related processes in the retina…
In addition to examining the brain, the researchers also explored how the gut microbiome’s age affects the retina. In general, it was shown that compared to young mice, aged mice had increased inflammation in the retina. However, after FMT, aged mice with young microbiomes had retinal inflammation levels similar to those of young control mice. In line with the findings in the brain, the opposite was also true. Young mice with aged microbiomes had retinal inflammation that resembled aged control mice. The gut microbiome also affects another part of the visual system: the photoreceptors’ ability to regenerate in the retina with the help of the RPE65 protein, whose production is also known to decrease with age (Cai et al, 2009). In aged mice with young microbiomes, there was an increased amount of the RPE65 protein compared to aged control mice. In fact, these protein levels were similar to the levels in young mice. Furthermore, young mice with aged microbiomes had much less RPE65 than young control mice, with protein levels comparable to levels seen in aged mice. Overall, this shows that the microbiome impacts age-related processes in the retina, with young microbiomes reversing and aged microbiomes accelerating processes associated with aging.
Another important organ, the intestines, are also not spared from the effects of aging: the cell layer that forms the wall of the intestines becomes leaky over time (Cui et al, 2019; Thevaranjan et al, 2017). Over the course of aging, the stability of the intestinal wall decreases and becomes more permeable, which allows bacteria to leak out into the periphery, which in turn increases overall inflammation (Cui et al, 2019; Thevaranjan et al, 2017). In this study, the researchers showed that the age of the microbiome affects the stability of the intestinal walls. In aged mice with a young microbiome, the intestines were less leaky than aged control mice. In fact, intestinal permeability in aged mice with a young microbiome was similar to the permeability seen in young mice. Aged mice with young microbiomes also had levels of inflammation and evidence of bacteria in the blood similar to young mice. Once again, the intestines of young mice with aged microbiomes behaved similarly to aged mice with aged microbiomes by having a leakier gut and more inflammation than young mice with young microbiomes. These results support the hypothesis that aged microbiomes contribute to the increase in intestinal permeability, which facilitates an increase in inflammation by allowing bacteria to leak into the blood stream. Importantly, introducing a young microbiome through FMT reverses these age-related effects.
…gut microbiome age impacts functions of the brain, retina, and gut.
The study’s results show that gut microbiome age impacts functions of the brain, retina, and gut. But how do young and aged microbiomes differ from one another? To answer this question, the researchers sequenced the DNA of the microbiome found in the fecal samples collected over the course of the experiment. The young and aged microbiome already had different genetic makeups before FMT occurred, but FMT significantly changed the genetic composition of both microbiomes. Young mice with aged microbiomes had a very similar composition to aged control mice, while genetic composition in aged mice with young microbiomes was different from aged control mice and also differed from young mice with young microbiomes – they were somewhere in between. Aged control mice and young mice with aged microbiomes had bacteria mostly from the Oscillibacter and Prevotella genus, Firmicutes phylum, and Lactobacillus johnsonii species, while young control mice and aged mice with young microbiomes had bacteria mostly from the Bifidobacterium, Ackermansia, Parabacteroides, Clostridium, and Enterococcus groups. When investigating the potential cause of these age-related changes, researchers found that pathways involved in lipid and vitamin production (which rely on metabolites produced by bacteria), differed between aged and young microbiomes. There’s one drawback to this observation – the changes in the abundance of different kinds of bacteria and their potential function in the gut was not long lasting, as there were no large differences between microbiome composition two weeks after FMT.
Overall, this study showed that the gut microbiome influences age-related processes in the brain, eye, and gut. Aged microbiomes, independent of the age of the recipient mouse, led to more inflammation in the brain, retina, and intestines, less regeneration potential in photoreceptors in the retina, and more bacteria leaking out of the intestines. On the other hand, introducing young microbiomes to aged mice reversed these aging effects. This may be due to the differences in the bacterial composition of aged and young microbiomes, and the effect that these alterations may have on pathways responsible for lipid and vitamin production. One question that wasn’t addressed in this study was how microbiome age affects cognitive performance, as neither control mice nor FMT mice behaved differently in behavioral memory tests. Future research should also focus on this question as cognition and memory are known to diminish with age, and understanding the role of the microbiome in age-related cognitive decline could provide important insight into possible biological underpinnings. Another direction that future research questions should pursue would be the impact of diet on the composition of the gut microbiome. Previous studies have shown that different diets alter the types of microbes in the gut both in the short-term (David et al., 2014) and long-term (Wu et al., 2011). If changes in diet can alter the composition of the gut microbiome, what if it can reduce these signs of aging in the brain, retina, and intestines too?
If changes in diet can alter the composition of the gut microbiome, what if it can reduce these signs of aging in the brain, retina, and intestines too?
About The Authors
Written by Holly Korthas, Illustrated by Federica Raguseo, Edited by Johanna Popp, Sarah Wade, and Lauren Wagner
References
Cai, X., Conley, S. M., & Naash, M. I. (2009). RPE65: role in the visual cycle, human retinal disease, and gene therapy. Ophthalmic Genetics, 30(2), 57–62. https://doi.org/10.1080/13816810802626399
Cui, H., Tang, D., Garside, G. B., Zeng, T., Wang, Y., Tao, Z., Zhang, L., & Tao, S. (2019). Wnt Signaling Mediates the Aging-Induced Differentiation Impairment of Intestinal Stem Cells. Stem Cell Reviews and Reports, 15(3), 448–455. https://doi.org/10.1007/s12015-019-09880-9
Czirr, E., & Wyss-Coray, T. (2012). The immunology of neurodegeneration. The Journal of Clinical Investigation, 122(4), 1156–1163. https://doi.org/10.1172/JCI58656
David, L., Maurice, C., Carmody, R. et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563 (2014). https://doi-org.proxy.library.georgetown.edu/10.1038/nature12820
Erny, D., Hrab? de Angelis, A. L., Jaitin, D., Wieghofer, P., Staszewski, O., David, E., Keren-Shaul, H., Mahlakoiv, T., Jakobshagen, K., Buch, T., Schwierzeck, V., Utermöhlen, O., Chun, E., Garrett, W. S., McCoy, K. D., Diefenbach, A., Staeheli, P., Stecher, B., Amit, I., & Prinz, M. (2015). Host microbiota constantly control maturation and function of microglia in the CNS. Nature Neuroscience, 18(7), 965–977. https://doi.org/10.1038/nn.4030
Heneka M. T. (2019). Microglia take centre stage in neurodegenerative disease. Nature Reviews. Immunology, 19(2), 79–80. https://doi.org/10.1038/s41577-018-0112-5
Hoh Kam, J., Lenassi, E., & Jeffery, G. (2010). Viewing ageing eyes: diverse sites of amyloid Beta accumulation in the ageing mouse retina and the up-regulation of macrophages. PloS One, 5(10), e13127. https://doi.org/10.1371/journal.pone.0013127
Ma, T. Y., Hollander, D., Dadufalza, V., & Krugliak, P. (1992). Effect of aging and caloric restriction on intestinal permeability. Experimental Gerontology, 27(3), 321–333. https://doi.org/10.1016/0531-5565(92)90059-9
Parker, A., Romano, S., Ansorge, R., Aboelnour, A., Le Gall, G., Savva, G. M., Pontifex, M. G., Telatin, A., Baker, D., Jones, E., Vauzour, D., Rudder, S., Blackshaw, L. A., Jeffery, G., & Carding, S. R. (2022). Fecal microbiota transfer between young and aged mice reverses hallmarks of the aging gut, eye, and brain. Microbiome, 10(1), 68.
https://doi.org/10.1186/s40168-022-01243-w
Sommer, F., & Bäckhed, F. (2013). The gut microbiota—masters of host development and physiology. Nature Reviews. Microbiology, 11(4), 227–238. https://doi.org/10.1038/nrmicro2974
Thevaranjan, N., Puchta, A., Schulz, C., Naidoo, A., Szamosi, J. C., Verschoor, C. P., Loukov, D., Schenck, L. P., Jury, J., Foley, K. P., Schertzer, J. D., Larché, M. J., Davidson, D. J., Verdú, E. F., Surette, M. G., & Bowdish, D. M. E. (2017). Age-Associated Microbial Dysbiosis Promotes Intestinal Permeability, Systemic Inflammation, and Macrophage Dysfunction. Cell Host & Microbe, 21(4), 455–466.e4. https://doi.org/10.1016/j.chom.2017.03.002
Wu, G. D., Chen, J., Hoffmann, C., Bittinger, K., Chen, Y. Y., Keilbaugh, S. A., Bewtra, M., Knights, D., Walters, W. A., Knight, R., Sinha, R., Gilroy, E., Gupta, K., Baldassano, R., Nessel, L., Li, H., Bushman, F. D., & Lewis, J. D. (2011). Linking long-term dietary patterns with gut microbial enterotypes. Science (New York, N.Y.), 334(6052), 105–108. https://doi-org.proxy.library.georgetown.edu/10.1126/science.1208344
This article originally appeared on Knowing Neurons