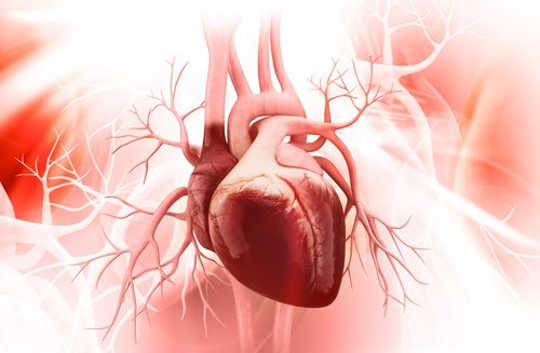
Doctors haven’t known why some babies are born with thin, spongy heart muscles. New research links the disease to poorly developed blood vessels around the heart.
Apart from a deeper understanding of congenital heart disease, the results could shed light on how heart muscle forms in the first place, say the study’s two senior authors, Ashby Morrison and Kristy Red-Horse, assistant professors of biology at Stanford University. Until now, they say, no one realized what an important role newly-forming blood vessels played in supporting the growth of heart muscle—or that the support is more than just a matter of supplying oxygen.
Red-Horse, who is a member of Stanford Bio-X, the Cardiovascular Institute, and the Child Health Research Institute, studies the development of tissues and whole organs, often by breeding her own genetically modified mice. Much of Morrison’s research, meanwhile, centers on the basic molecular machinery that reads out messages in the DNA and uses it to build functioning cells—usually in yeast.
Their neighboring offices got them talking, and among the topics of conversation was a particular molecule that Morrison had been looking at, one that turns out to be present not just in yeast but also in mice and many other living things, too. That got them wondering: what did that molecule do in those other living things, and what would happen if it disappeared?
In yeast, the molecule, called Ino80, is pretty important—without it, yeast get sick and die off—but in other organisms, “we didn’t know what to expect,” says Morrison, a member of Stanford Bio-X, the Child Health Research Institute, and the Stanford Cancer Institute.
Custom mice
To find out, Red-Horse and her lab started the years-long process of genetically modifying mice to lack Ino80, either throughout their bodies or in specific areas of the body or specific cell types.
The most intriguing results, Red-Horse says, came from mice which didn’t produce Ino80 in certain heart cells—called endothelial cells—that are the progenitors of blood vessels that feed the muscles of the heart. Without Ino80, the network doesn’t develop properly, and as a result, cardiac muscles couldn’t develop properly either—instead remaining spongy and weak.
It was at this point that the team noticed the similarity between their mice and a form of heart disease called left ventricular non-compaction, the third most common disease of the heart muscle. “It was a complete surprise,” Morrison says.
The missing factor
Curiously, blood flow through those missing vessels—and the oxygen it provides—is only part of the story. In a follow-up experiment, the researchers grew heart muscles in a dish along with endothelial cells that had not yet formed into blood vessels. The team found that when those endothelial cells produced no Ino80, the heart muscle didn’t develop properly. Apparently, Red-Horse says, “endothelial cells are producing something that’s a growth factor” for cardiac muscle cells. “The next step is to identify that factor.”
Still, what they’ve found already should change how both doctors and biologists think about how the heart forms, says Red-Horse.
In both cases, taking into account the role of blood vessels could help explain normal muscle development in mice and then humans or lead to new therapies for diseases like left ventricular non-compaction. Farther down the road, the research could also have implications for regenerative medicine specialists working to grow hearts and other organs in the lab, Red-Horse and Morrison say.
Funding came from the National Institutes of Health, the American Heart Association, the National Science Foundation, and the Burroughs Wellcome Fund. The findings appear in Nature Communications.
Source: Stanford University
Related Books:
at InnerSelf Market and Amazon