Illustration by McCall Sarrett
Whether it be forgetting 20 years of your life or having the same conversation every five minutes only to forget it each time, memory impairment can take a large toll on everyday life. It can be one of the most confusing aspects of neurodegeneration and is a key symptom of dementia.
Alzheimer’s Disease, also known as AD, is the most common form of dementia, characterized by cognitive difficulties and memory loss. There is currently no official clinical technique for diagnosing AD, other than an autopsy. However, there are two characteristic features of the brain tissue of Alzheimer’s disease patients: amyloid beta (A?) plaques and neurofibrillary tangles, both of which have provided a substantial amount of insight into the pathology of neurodegeneration.
“Neurofibrillary tangles arise from a defect in proteins called tau proteins.”
A? is a protein that results from proteolysis of Amyloid Precursor Protein (APP). This means that APP is cut into smaller fragments, one of which is the A? fragment. APP is cut into these pieces by enzymes called secretases, whose primary role is to cleave these proteins. Various secretases exist, but there is one that is significant for Alzheimer’s pathology – gamma secretase. Gamma secretase produces a special form of A?: A?-42, the most toxic form of the protein. Once broken up, these protein fragments begin to gather in the space outside of the cells. The key to the toxicity of these proteins is that they are characteristically “sticky”, so they begin to form aggregates. These aggregates continue to develop, and soon these amyloid beta plaques are everywhere, greatly impairing neuronal function.
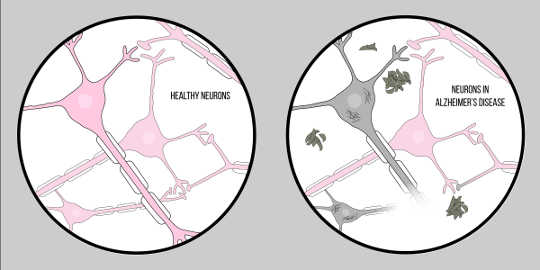 Figure by McCall Sarrett
Figure by McCall Sarrett
This impact of A? on neurological function has led to the Amyloid Hypothesis, a well recognized theory proposing an explanation for the neurodegeneration in Alzheimer’s.
Now, for the second key characteristic: neurofibrillary tangles. Neurofibrillary tangles arise from a defect in proteins called tau proteins. Tau proteins serve as a bridge between structures called microtubules within the cell. Microtubules are supporting molecules which provide shape and structure to the cells, specifically, axons. Tau proteins help these microtubules provide structure to the axons. However, in the case of Alzheimer’s diseaseA neurodegenerative disease characterized by neuronal loss i..., tau proteins separate and accumulate in the cell body, otherwise known as the soma. This causes degeneration of the axons, making it even more difficult for neurons to communicate. This difficulty in neuronal communication is similar to the effects of amyloid beta; however, it is important to note that these tangles differ greatly from the plaques discussed previously in that they influence communication from inside of the cell as opposed to outside.
“Scientists are still unsure of the exact mechanism responsible for the pathology of Alzheimer’s.”
Though both are extremely influential, A? and tau are not the only relevant factors when it comes to the pathology of Alzheimer’s. Studies have suggested that the Apolipoprotein E (APOE) gene may be as influential as A?. There are three major variants that encode for proteins: ApoE2, ApoE3, and ApoE4. ApoE4 has been shown to correlate with a decrease in synaptic pruning, while ApoE2 results in an increase. Both forms influence astrocytes (important glial cells within the nervous system) and are correlated to their rate of phagocytosis, the process by which the astrocytesStar-shaped glial cells that have a number of functions, inc... engulf cellular material. Each ApoE variant directly influences this rate of phagocytosis, ApoE2, resulting in an increase, and ApoE4, a decrease. This suggests that the astrocytes may be less able to “clear out” the debris floating around in the cell when correlated with ApoE4. Thus, when these plaques build up, the ApoE4 genotype may prevent clearance of these aggregates, contributing to degeneration.
Amyloid beta, tau, and various genes all work together to cause a major communication issue between cells, which is essentially the disease we know as Alzheimer’s. Such neurodegeneration is most common in areas of the brain associated with learning and memory, but eventually spreads throughout the entire brain. With this lack of communication comes a loss of synapses, and eventually, a decrease in brain matter. Thus, it is normal to see decreased brain tissue on an MRIMagnetic resonance imaging, a technique for viewing the stru... as the disease progresses, revealing that Alzheimer’s literally shrinks the brain. Our current models of learning and memory state that synapses play a key role in these processes, providing a possible explanation as to how this pathology correlates to the major symptoms of AD, such as memory loss.
Scientists are still unsure of the exact mechanism responsible for the pathology of Alzheimer’s. Many innovative methods have been researched in an attempt to gain further knowledge of this pathological point, one being cerebrospinal fluid (CSF) biomarkers.
Levels of A? and tau can be measured in CSF through a procedure called a lumbar puncture, which collects cerebrospinal fluid. Increased levels of tau and reduced levels of A? are seen in patients with Alzheimer’s Disease. This is a result of the A? accumulations in the brain, which in turn results in a decreased concentration in the CSF. This data suggests that, in the future, doctors and scientists may be able to predict the cognitive state of a patient by examining protein levels in the CSF.
Currently, there is no definitive cure for Alzheimer’s disease. Yet increasing knowledge of the pathological proteins involved, associated genes, and ongoing scientific research provide hope for an effective treatment in the future.
What breakthrough do you think are needed for medicine to achieve a treatment for Alzheimer’s? Let us know in the comments!
This article originally appeared on Knowing Neurons?
About The Author
Khayla Black is a freshman at New York University Shanghai planning to declare a major in Neural Sciences with a minor in Data Science with a concentration in artificial intelligence. In the future, she hopes to obtain an MD/PhD and become a neuroscientist studying the molecular aspects of learning and memory. During her spare time, Khayla enjoys working with the MYELIN initiative within IYNA as well as reading any neuroscience related material. Outside of studying neuroscience, she enjoys running, teaching at local elementary schools, solving math problems, and drinking immense amounts of coffee.
Related Books
at InnerSelf Market and Amazon
References
Lacor, P. N., Buniel, M. C., Furlow, P. W., Clemente, A. S., Velasco, P. T., Wood, M., . . . Klein, W. L. (2007, January 24). A? Oligomer-Induced Aberrations in Synapse Composition, Shape, and Density Provide a Molecular Basis for Loss of Connectivity in Alzheimer’s Disease. Retrieved from http://www.jneurosci.org/content/27/4/796
Murphy, M. P., & LeVine, H. (2010). Alzheimer’s Disease and the ?-Amyloid Peptide. Journal of Alzheimer’s Disease?: JAD, 19(1), 311. http://doi.org/10.3233/JAD-2010-1221
O’Brien, R. J., & Wong, P. C. (2011). Amyloid Precursor Protein Processing and Alzheimer’s Disease. Annual Review of Neuroscience, 34, 185–204. http://doi.org/10.1146/annurev-neuro-061010-113613
Chung, W.-S., Verghese, P. B., Chakraborty, C., Joung, J., Hyman, B. T., Ulrich, J. D., … Barres, B. A. (2016). Novel allele-dependent role for APOE in controlling the rate of synapseConnections between neurons where a signal is passed from on... pruning by astrocytes. Proceedings of the National Academy of Sciences of the United States of America, 113(36), 10186–10191. http://doi.org/10.1073/pnas.1609896113
Zetterberg, H. (2009, August 19). Amyloid ? and APP as biomarkers for Alzheimer’s disease. Retrieved from https://www.sciencedirect.com/science/article/pii/S0531556509001594?via=ihub